When you were a child and fell playing outside, a single bandage — a placebo of sorts — could take away the pain.
The placebo effect has long been somewhat of a medical mystery. While researchers found it triggers a chemical reaction in the brain about 40 years ago, how a sugar pill can ease a person’s pain has not been fully understood by scientists.
Until now.
A study recently published in the journal Nature, co-authored by a Harvard scientist, revealed what physically occurs in the brain to trigger the placebo effect — and where in the brain it surprisingly occurs.
Researchers say this study could lead to the development of novel, non-addictive and non-opioid treatments for pain.
The findings were made possible in part through the work of postdoctoral Harvard Medical School fellow, Andrew Shuster, who genetically engineered mice in 2013 that were pivotal to the research. He had been a Stanford University lab manager and technician at the time, just after graduating with an undergraduate degree.
“This is a project we started a long time ago,” said Greg Scherrer, who headed the Stanford lab and was an author on the study since its start. Scherrer is now an associate professor of cell biology and physiology at the University of North Carolina Chapel Hill.
His lab, Scherrer Lab, is interested in better understanding pain experience and how opioids relieve pain, but also how they produce harmful side effects such as addiction.
The mice Shuster genetically engineered were being used for opioid-related neuroscience projects, Scherrer said.
How the brain reacts to pain during the placebo effect
By using the critters, the team was able to examine placebo analgesia, also known as the natural painkilling that occurs when the placebo effect takes place.
They also saw how the placebo effect causes a reaction in the brain that is similar to that produced by opioids.
“The idea [with this study] is to better understand how our brain uses systems that are already present in our body to modulate our pain thresholds,” Scherrer said.
He explained one of those existing internal systems is called endogenous opioids. These are peptides naturally released by the body’s cells in certain situations, such as the placebo effect and stress, to change how we perceive pain.
The effect of endogenous opioids is comparable to “a low dose of oxycodone or morphine,” Scherrer said.
And just as naxolone, commonly known as Narcan, can stop the overdose effects of opioids such as fentanyl, morphine or oxycodone in the emergency room, Scherrer said the effects of the endogenous opioids can be stopped by naxolone during the placebo effect.
This shows a connection between both natural and artificial opioids, which each act as painkillers.
“We’ve known since studies in the 1980s that when we expect pain relief, our brain is able to anticipate and reduce the perception of pain — even if you are given a sugar pill, a fake treatment,” Scherrer said.
“But since [the 1980s], not much progress has been made in terms of [determining] the particular neuroscience in the brain, or cell types that produce this possible response,” he said.
That information could lead drug companies to start the lengthy process of making a new, non-opioid, and non-addictive painkiller drug based on how endogenous opioids reduce pain during the placebo effect.
Mice study reveals what happens in brain – and where it happens – during placebo effect
To find out what’s happening in the brain during the placebo effect, the scientists recreated the phenomenon by conditioning a group of Shuster’s mice.
The mice were put in an enclosed environment that had two different spots, which Scherrer called “plates.” The first plate was always heated to 118 degrees, while the second was always at 86 degrees.
After some time, the mice became accustomed to each plate’s heat and would continually choose to sit on the more comfortable second (86-degree) plate.
But when the second plate was heated to the same 118 degrees and the mice were offered both, the scientists found the mice continued to prefer the second plate despite it being just as hot at 118 degrees.
The mice did not show signs of discomfort, such as licking, rearing and jumping, as they did when they came in contact for the first time with high 118-degree temperature.
After inducing the placebo effect on the mice, the scientists studied which parts of the brain were activated during the effect. They also found mice that were not conditioned — control mice — showed more signs of discomfort with the heat than those under the influence of the placebo effect.
Most intriguing to the team was the discovery of a neural circuit pathway between different parts of the brain. The pathway was through the rostral anterior cingulate cortex (rACC), which controls the processing of emotional and cognitive information, the pontine nuclei (Pn) in the brainstem, and the cerebellum, which controls complex motor functions.
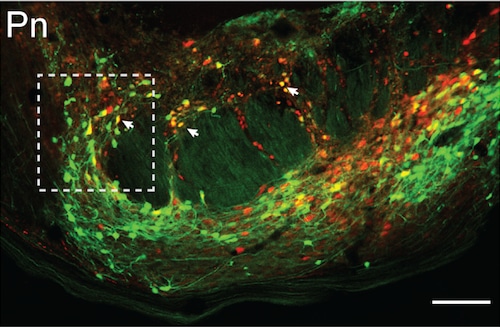
Neurons in the pontine nucleus (Pn) travel in the neural circuit tracing experiment to produce placebo pain relief done by Chong Chen, using mice engineered by Andrew Shuster. (Image courtesy Greg Scherrer)Greg Scherrer
This was a surprise to researchers because both the cerebellum and Pn had not been recognized as part of the network of brain areas that usually respond to and process pain, known as the pain matrix. The cerebellum has been previously linked to pain, but researchers said its role remains “mysterious.”
As the cerebellum is the main target of Pn neurons, the scientists looked at that part of the brain while the placebo effect was happening. They found a cluster of cells encoded by the expectation of pain relief and found that the cluster’s activity was driven by the rACC-to-Pn neural circuit pathway.
While not the first study on animals with the placebo effect, this is the most in-depth explanation of what happens in the brain during the placebo effect published to date, according to the journal Nature.
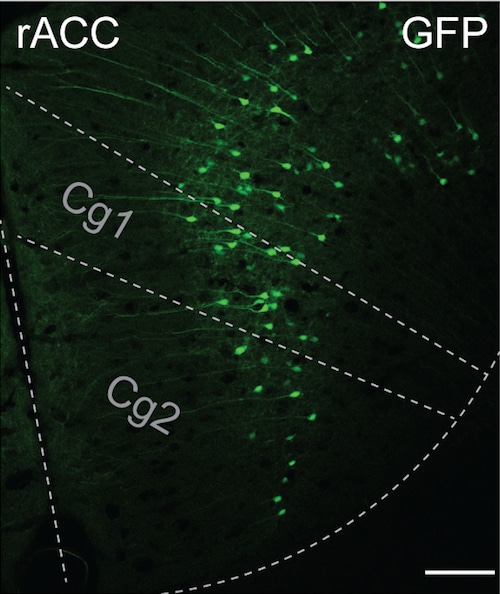
The rostral anterior cingulate cortex (rACC) in the brain receives information from cells in the pontine nucleus to produce placebo pain relief in the neural circuit tracing experiment done by Chong Chen with mice genetically engineered by Andrew Shuster. (Image courtesy Greg Scherrer)Greg Scherrer
Titled “Neural circuit basis of placebo pain relief,” the study listed Chong Chen as the primary of 14 total authors. An article on the study by Jeffrey Mogil called “Placebo effect involves unexpected brain regions” was also published in Nature on Aug. 21.
‘Our brain is very good at fixing its own problem of pain’
“This is the starting point. It’s really that, somehow, our brain is very good at fixing its own problem of pain,” Scherrer said.
“It’s a very real and potent effect, and so that’s what motivated us to study this system — because if you can find the cells that do this naturally, there might be therapeutic ways to engage these circuits or these cells and make them function even better, to develop a novel generation of treatments for pain,” he said.
Though further work remains to be done on how these discoveries in mice compare with humans’ more complex systems, Scherrer explained that the next step in the drug discovery process will be to identify the target in these cells and create an “asset” to produce the desired effect (painkiller) from the target.
“The placebo effect is a big deal, and it’s a big step for the field to get this knowledge about the [brain] circuitry that’s responsible. As far as our mission is concerned, we’ve done our part of the job … but there’s always more you can understand,” Scherrer said. His work in pain and opioids will continue, he said.
Since his time working with Scherrer, Shuster has kept his focus on neuroscience using mice as a model organism and has been published as a co-author numerous times over the past decade.
At Harvard, he is back with his “true love” researching somatosensory neurons, or cells in the body that detect senses such as pain or warmth. He said this project was one of the starting points for his current career.
“Greg got me interested in this field, so I have Greg to thank for introducing the field to me and being a very good mentor back in the day,” Shuster said. He added it had been an “exciting time” learning about the field and contributing to it, especially on “what I felt and what we all felt was a very important and very interesting set of projects.”






